Chemical Properties of Acids and Bases
Chemical Properties of Acids and Bases: Overview
This topic covers concepts, such as, Chemical Properties of Acids, Reactions of Acid with Metals, Reactions of Non-metallic Oxides with Bases & Nature of Non-Metallic Oxide etc.
Important Questions on Chemical Properties of Acids and Bases
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) The temperature of the solution increases.
(ii) The temperature of the solution decreases.
(iii) The temperature of the solution remains the same.
(iv) Salt formation takes place.
Metallic oxides are ______ in nature, but non-metallic oxides are ______ in nature. The information in which alternative completes the given statement is:
The gas evolved when aluminium powder is mixed with sodium hydroxide solution is:
The chemical used in fire extinguishers is
The chemical equation that represents neutralization reaction among the following is
Which substance when used with butter having butyric acid can cure acidity
A diagram of the reaction of Zinc with acid is given below.
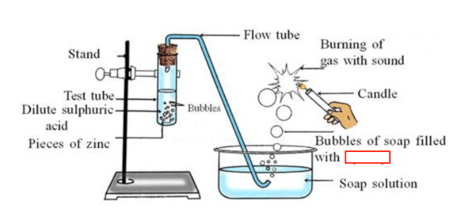
During this reaction, a gas is evolved. Write the name of the gas. (Sulphur dioxide/ Hydrogen)
_____ solution is used to remove sulphur dioxide, oxides of nitrogen, etc.
Lead nitrate solution is used to remove _____.
_____ solution is used to remove arsine and phosphine.
_____ zinc is used in the preparation of hydrogen gas.
Which of the following elements will form an acidic oxide?
Which of the following gas turns lime water milky?
The oxides formed by metals are _____ in nature.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O is the example of a _____ reaction.
The reaction between an acid and a base to give a salt and water is known as a _____ reaction.
What should be the atomic number for an element to form an acidic oxide?
Metal is heated with dilute sulphuric acid H2SO4. The gas evolved is collected. The gas is _____.
Acids react with bases to form _____ and water.
When magnesium and hydrochloric acid react, they produce_____________.
